Published 2022-08-09.
Time to read: about 3 minutes.
How Do Salts Dissolve?
The atomic structure of salt crystals form a lattice, which is composed of alternating positive and negative ions.
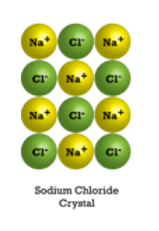
Water dissolves ionic compounds such as salts, partly because water is a polar molecule. Polar molecules have an asymmetric geometry, which causes them to have an uneven electric charge around them.
When a crystal of salt is placed in water, the water molecules collide with the salt’s crystal lattice. Water is attracted to the sodium chloride ions in the crystal lattice because water molecules have positively charged and a negatively charged regions. The positively charged sodium ions in the crystal attract the oxygen ion in water molecules, which are negatively charged. The negatively charged chloride ions in the crystal attract the hydrogen ends of the water molecules because they are positively charged.
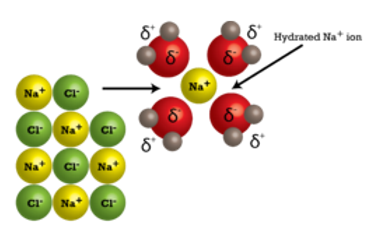
When salt dissolves, its ions become surrounded by water molecules. As water molecules bump into the salt’s ions, the crystal lattice starts coming apart.
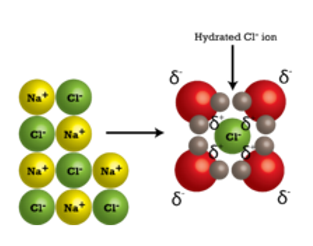
After coming apart from the salt crystal, the individual Na and Cl ions are surrounded by water molecules. The individual sodium (Na+) ions are surrounded by water molecules with the oxygen atom oriented near the positive ion. Likewise, the chloride (Cl-) ions are surrounded by the hydrogen ions in water molecules, which have the opposite charge.
Portions of this section were based on CC licensed material published by Chemistry Concepts Intermediate.
Authored by: Calbreath, Baxter, et al..
Provided by: CK12.org.
Located at: http://www.ck12.org/book/CK-12-Chemistry-Concepts-Intermediate/.
License: CC BY-NC: Attribution-NonCommercial
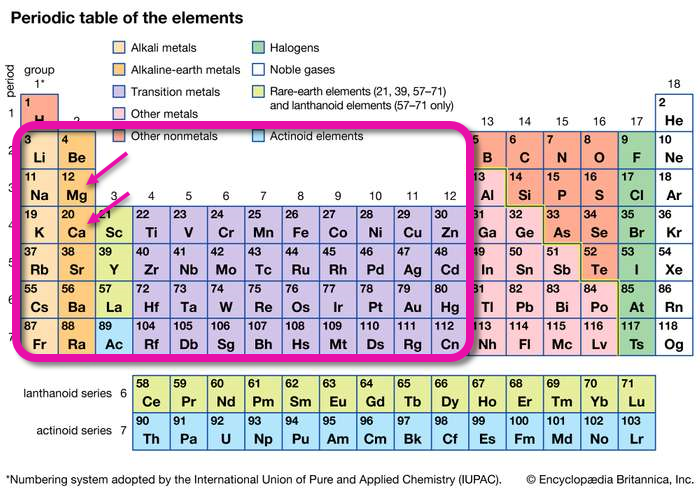
Elements That Make Water Hard
Hard water ions come from groups 1 through 12 on the period table of elements.
The rounded rectangle encloses all of the elements that make water hard when they dissolve. The arrows point to elements Mg (magnesium) and Ca (calcium). Other elements within the rounded rectangle can also make water hard; for example, Fe (iron) and Na (sodium) are found in water from some areas.